Database Lock In Clinical Trials Ppt
Center for Drug Evaluation and Research. Introduction One of the most crucial aspects of research is clinical data management or CDM.
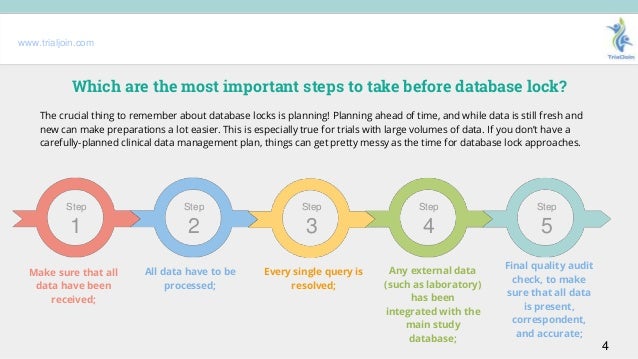
Explaining The Importance Of A Database Lock In Clinical Research
Explaining the importance of a database lock in clinical research 1.

Database lock in clinical trials ppt. AE Adverse EventAn undesirable medical condition to a subject during a clinical trial. Issue at the source but that is not always feasible. Most clinical trials will normally run for a long period of time and it is important that the latest dictionary is used and that appropriate plans are in place to.
Re-locking the database should follow the same process for notificationapproval as the initial lock. Request supporting information from the vendor or site manifest enrollment log etc and work with the clinical team to resolve the discrepancy between the eCRF data and the non-eCRF data Once the discrepancies have been reconciled the two data sources are integrated into the official database of record used for analysis purposes. Clinical Data Management CDM is a critical phase in clinical research which leads to generation of high-quality reliable and statistically sound data from clinical trials.
Additionally today interim analyses or analyses for safety review are more and more common and hence the teams need to work on unclean or semi-clean data. Also note that the terms clinical trial and study are used interchangeably throughout this paper. Clinical Data Management is the collection integration and validation of clinical trial data During the clinical trial the investigators collect data on the patients health for a defined time period.
A trial data manager plays a key role in achieving successful database lock by acting as liaison between statisticians clinical and technical personnel and ensuring that every step related to data collection and verification was followed properly. Clinical data archiving includes planning implementing and maintaining a repository of documents andor electronic records containing clinical information supporting documentation and any interpretations from a clinical trial. Due to the effort that goes into locking a database and the effort that would be required to re-open the data for editing the most effective solution to fixing data issues is to.
Database lock Awareness of. This data is sent to the trial sponsor who then analyzes the pooled data using statistical analysis. Examination of trial related activities and documents to determine whether the evaluated trial related activities were conducted and the data were recorded analyzed and accurately reported according to the protocol sponsors standard operating procedures SOPs Good Clinical Practice GCP and the applicable regulatory requirements.
The lock of a database is a very important milestone in a study and once the final database lock has occurred no further changes can be made without special permission. A career in Clinical analysis offers glorious job opportunities in the USA Europe Singapore. MOTIVATION It is a given that a variety of statistical and programming tasks are going to take place before database lock.
Explaining the Importance of a Database Lock in Clinical Research 2. AFTER THE DATABASE LOCK FOR THE FINAL ANALYSIS1 1. This helps to produce a drastic reduction in time from drug development to marketing.
This SOP is primarily to provide higher level information for Trial database locking. Clinical analysis is remarked the whole listing of a drug device or biological take a look at. Before explaining the taboo behind Clinical Database Unlock let me introduce you with few terminologies.
Data is also a key factor to consider during trial close-out. Clinical Registries While RCTs test hypotheses the real world of clinical practice is a registry. A good example of this in clinical trials is a data issue that is found after database lock DBL.
All patients generalizability Dynamic timeliness Significant Potential cost savings when automated clinical registry database system bundled with other functional requirements clinical reporting billing inventory control. CRAs should collaborate directly with data management to ensure data is monitored and cleaned on an ongoing basis. Independent of how individual companies perform these tasks within their company each company is obligated to ensure that the.
BEVERLY MAMarketwired April 19 2016 - Cellceutix Corporation OTC. This can present problems given the nature of the data that are. CTIX the Company a clinical stage biopharmaceutical company developing innovative therapies with oncology dermatology anti-inflammatory and antibiotic applications is pleased to inform shareholders that the clinical database for Prurisols Phase 2 FDA trial for mild-to-moderate chronic plaque psoriasis.
Common Challenges in Conduct of Clinical Trials Rahnuma Wahid PhD Technical and Project Coordinator Vaccine Development March 17 2015 8th Meeting with International Partners for Influenza Vaccine Technology Transfer to Developing Country Vaccine Manufacturers. Database Lock means BCC s receipt of written notice from the CRO responsible for the Current Phase 3 Clinical Trials that all Data required by the Protocols has been entered into the database reviewed queries resolved and issues addressed and that such Data is. However the primary focus from a clinical operations perspective is data cleaning to meet the database lock milestone.
Throughout the course of a clinical trial. India goes to be the key hub of clinical trials. Mandated to do so.
A database is locked after review query resolution and determination that it is ready for analysis. Statistical checks for database acceptance before database lock of a clinical trial are not widely used. Patients who require continuation of treatment within this trial after the database lock for the final analyses will have reduced number of trial visits and reduced trial related procedures as described in this flow chart.
INTRODUCTION The process of locking a clinical trial database is an action taken to prevent further changes to the database. PowerPoint PPT presentation free to view. Design clinical trials database design and programming data acquisition and entry into the clinical trials database data review validation coding and database finalization.
Proper CDM will generate. Conducting Clinical Trials During the COVID-19 Public Health Emergency Jacqueline Corrigan -Curay MD JD Director Office of Medical Policy. Database lockunlock - This is a controlled procedure to freeze data to prevent writeedit access to users of the system.
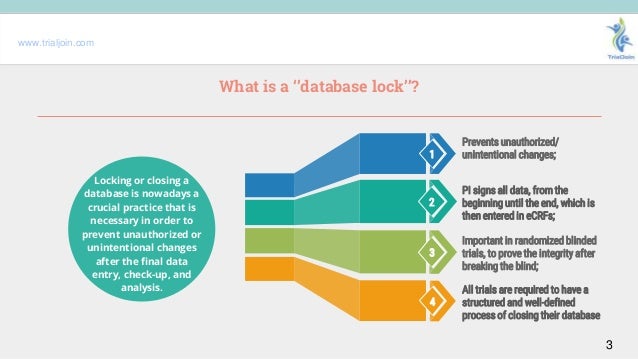
Explaining The Importance Of A Database Lock In Clinical Research

Competency Framework For The Clinical Research Professional Clinical Research Research Clinic

Institutional Review Boards Irbs Are Specialized Committees Required By Hhs Regulations That Safeguard The Rights And W Review Board Research Clinical Trials
Posting Komentar untuk "Database Lock In Clinical Trials Ppt"