What Happens After Database Lock In Clinical Trials
Phase IV clinical trials happen after the FDA has approved medication. This however requires proper documentation and an audit trail has to be maintained with sufficient justification for updating the locked database.

Competency Framework For The Clinical Research Professional Clinical Research Research Clinic
Certainly you could have a 100 SDV in a NIS and perform the data cleaning for your interim analysis as you would for a clinical trial ie.

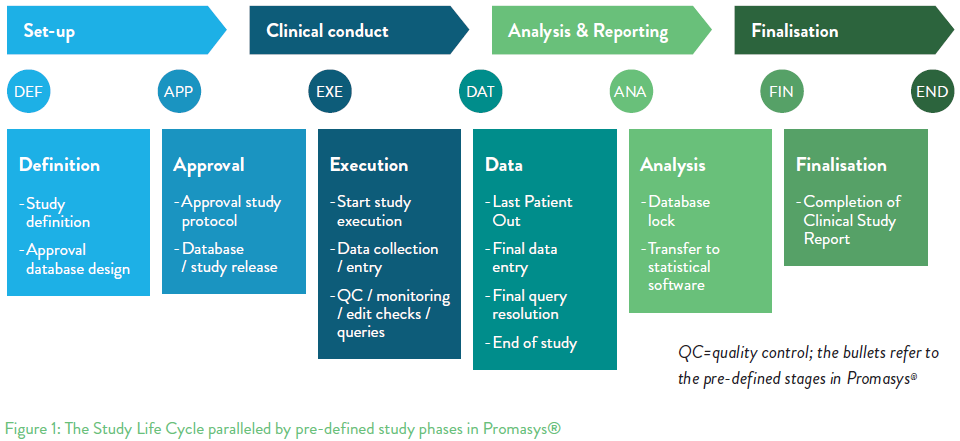
What happens after database lock in clinical trials. After a phase 1 or phase 2 trial they decide whether to continue testing the treatment. This paper exploresthe implications of working with this dataand it presents tools and processes that can reduce their impact. A database is locked after review query resolution and determination that it is ready for analysis.
After the lock an audit may be performed on the final data. Makes things easier at the end of the study. The Charity shall provide the Final Report to the Company and CRT within after the Clinical Trial Database Lock Date.
Clinical data archiving includes planning implementing and maintaining a repository of documents andor electronic records containing clinical information supporting documentation and any interpretations from a clinical trial. The database cannot be changed in any manner after locking and its important to ensure all pre-lock activities are checked off. Reconciling the databases to be sure that the clinical trial AEs are the same in the two databases is usually done periodically during the clinical trial rather than waiting till the end of the trial.
At the end of the trial prior to analysis Priment. The team then prepares for further analysis interpretation and reporting of findings in a clinical study report CSR. The clinical trial team reviews the dry run outputs which provide a glimpse of the final outputs.
Promotes prompt data entry. Once the database passes QA a QA certificate is generated documenting the actual error rate and hard lock can then be declared. After a clinical trial is completed the research team carefully analyzes information collected during the study to make decisions about the findings and any need for further testing but the next steps can vary based on what phase of testing the trial was in.
Any comments from the team on dry-run outputs are addressed and applied to the database for a final round of cleaning. This corrects errors in near real-time and avoids late expedited reports to health authorities. Statistical checks for database acceptance before database lock of a clinical trial are not widely used.
There are a number of instances when this is necessary. Once hard lock is declared all edit rights including those of the Lead Data Manager are rescinded from the team. Database lock A clinical trial term of art for an action taken to prevent further changes to the trial database.
A database is locked after review query resolution and determination that it. Syndax shall provide the Final Study Report to Genentech within six 6 months after Database Lock. A good example of this in clinical trials is a data issue that is found after database lock DBL.
Promotes entering data consistently and regularly. Within the conduct of a clinical trial there are many programming tasks that must be completed before data have been finalized and databases are locked. But post-trial care doesnt end there and.
Issue at the source but that is not always feasible. Prior to the final database lock a soft lock is declared. Data managers clinical manager database programmer and statistician are required to finalize the database lock.
Once the database has been completed signatures of responsible individuals eg. Due to the effort that goes into locking a database and the effort that would be required to re-open the data for editing the most effective solution to fixing data issues is to. What happens after the clinical trial is over is a valid enough enquiry even if you end up deciding not to participate in one.
But in case of a critical issue or for other important operational reasons privileged users can modify the data even after the database is locked. When all clinical trial data has been reviewed queries resolved and issues addressed then the database will be locked. After a clinical trial is over what most of you want to know is whether now youre entitled to the investigational drug or procedure if it proved to be beneficial to you.
Generally no modification in the database is possible. However this is an unrealistic scenario in the vast majority of NIS in a study with thousands of patients it is likely that dealing with data unlock-requests would be more than a full-time job. The process of locking a clinical trial database is an action taken to prevent further changes to the database.
At this point final tables figures and listings can. 9 Gives a more organized database at all times. Even though having a quarterly database lock might put some pressure on people responsible for this it also pushes them to enter their data promptly and on time.
Additionally today interim analyses or analyses for safety review are more and more common and hence the teams need to work on unclean or semi-clean data. Re-locking the database should follow the same process for notificationapproval as the initial lock. In order to prepare for an interim or final analysis of a clinical trial the datasets to be analysed must be finalised.
Most of the sponsors ensure a clean database before the final milestone of database lock which happens after the clinical trial team decides on database freeze. This phase involves thousands of participants and can last for many years. Lock the data and unlock upon request only.
First as noted above the available data on the time to publication after completion of a clinical trial suggest that the percentage of trials published increases continuously over time with a leveling off between 40 and 60 months after completion.
Orchestrated Clinical Trials Iqvia

Posting Komentar untuk "What Happens After Database Lock In Clinical Trials"