Arisg Safety Database
FAERS VAERS online datasets for safety monitoring and online filing of AEs. Click for the safety report that you want to reclassify.

Oracle Argus Safety Database Solutions Kvs Health
Aggregate report generation submission and distribution.

Arisg safety database. Find Top Paid Free online arisg safety database training courses certifications trainings programs specialization at Naukri Learning. Horizon 3 Drug Safety Associate I Resume Objective. Pharmacovigilance Electronic Safety Database Services.
The database owner may maintain the system on their servers or install the full system on a company server and allow access through licenses. The Re-Classification page appears. The Import Section page E2B R2 tab appears.
Argus Safety Argus Safety migrations Aris Global migrations ARISg to Argus migration Clintrace migrations drug safety system Empirica Trace migrations ETL Migration Oracle pharmacovigilance system safety database XML. LifeSphere Safety is an end-to-end drug safety platform that helps hundreds of pharmacovigilance teams around the world save time and effort ensure future-proof compliance and unlock deep insights from safety data. See a skim-through of our Argus Safety Database training and free guidesresources below.
Adverse Reaction Information System Global is one option -- get in to view more The Webs largest and most authoritative acronyms and abbreviations resource. Page 18 - Find Top Paid Free online arisg safety database training courses certifications trainings programs specialization at Naukri Learning. End to end pharmacovigilance services including receipt processing and scientific analysis submission and distribution of Individual Case Safety Reports ICSRs Global and local literature management.
PPD was able to assign a lead safety specialist for each report who compiled the data and wrote all sections of the report. To reclassify a case. Alternatively access to a safety database can be provided though a CRO or safety niche provider.
From the ARISg menu click ESM E2B import section Import Section. Lower cost and complexity through SaaS deployment. This situation in Safety platforms space was not achieved overnight but rather over 5 7 years through market consolidation.
Looking for the definition of ARISG. Aris Globals LifeSphere Safety otherwise known popularly as ARISg is another market-leading pharmacovigilance database with a long and well-established heritage in the industry. ARISg forms a core component of an integrated pharmacovigilance and risk management system enabling companies to monitor their products and identify safety risks proactively.
Efficient case processing via automations and ease of use. Implementation and maintenance- iSafety implementation services for Oracle ARGUS Safety Database are strategically tailored to support customers necessities in meeting their regulatory obligations for their pharmacovigilance ICSR Individual case safety reports process. More than Clinical I would say Safety is probably a better in example to highlight this situation where youd most likely find 2 products ruling the roost in Oracle Argus and Aris Globals ARISg LifeSphere Safety.
ARISg facilitated quick and efficient generation of periodic reports within the system based on industry standard reporting requirements. We have the experience of using the pre-validated Aris Global safety database ARISg a world leading Pharmacovigilance and clinical safety system. A Global Pharmacovigilance Safety Database sits at the heart of the vigilance system for medicinal products and devices.
It is the main repository for collected safety information for the companys products it facilitates the reporting of individual and aggregate safety data to authorities and third parties and provides a key source of information for ongoing detection of safety. The future of pharmacovigilance. Single global database supporting the drug safety requirements of multiple regions with multilingual capabilities including English Chinese Japanese and French.
Download this whitepaper to learn about LifeSphere Safety MultiVigilance. About the Author. With the adoption of ICH guidelines for clinical safety and pharmacovigilance reporting by.
To obtain a Drug Safety Associate position that will promote growth stability and opportunity for advancement and has experience in responsible for overseeing specific. Compliance with global regulations and standards. This may be a more attractive solution financially for limited case volumes.
For post-marketing drug safety surveillance Bioclinica provides. Without a reliable complaint and practical safety data base pharmacovigilance services simply wouldnt work in real life. Compare best arisg safety database training courses online from top Platforms Universities.
Well help you stay ahead. Rodney Lemery specializes in the implementation support. The case is imported as Initial and CNAN of the ICSR file is not imported to ARISg database.
Designated Medical Event PV Signal. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators. This offers the functionality to report and manage adverse events for drugs vaccines biologicals devices and combination products from case entry medical review coding via the integrated MedDRA browser to automatic.
Compare best arisg safety database training courses online from top Platforms Universities. The only pharmacovigilance and safety compliance solution with an in-built Chinese interface on the market. Powerful extensible and user-friendly analytics and reports.
ARISg helps speed up the management of Adverse Drug reactions with the use of its configurable workflow and advanced automation features. Arisg safety database training. Safety databases expedite the reporting of individual and aggregate safety data to authorities and third parties and provide critical information for detecting safety signals and the ongoing evaluation of the risk-benefit profile of the companys products.
Pharmacovigilance work should be an exercise in accuracy timeliness and compliance and the software the safety database uses literally underpins all the other work staff perform. MultiVigilance formerly ARISgARISc China is aligning its regulatory environment with that of global standards with an aim to promote innovation simplify drug development processes and to enhance the efficacy quality and safety of the drugs approved. A PPD safety physician then provided medical review of the reports.
Single global database that includes Japan cases and user interface. Oracle Argus Safety Certification is a 2 day 6-module program that covers every tab and task needed to manage drug safety data when using the Oracle Argus Safety Training application. Oracle Argus Safety Essentials The main essential features of Argus safety database.
This guide to some key features of the ARISgTM safety database can help you. It provides comprehensive suite of functionality and tools as well as additional modules and interfaces covering other aspects of pharmacovigilance as well as regulatory medical affairs and clinical.
Argus Software Medical Clinical Research Blog Certified Clinical Research Professionals Society Clinical Research Certification
Argus Software Medical Clinical Research Blog Certified Clinical Research Professionals Society Clinical Research Certification
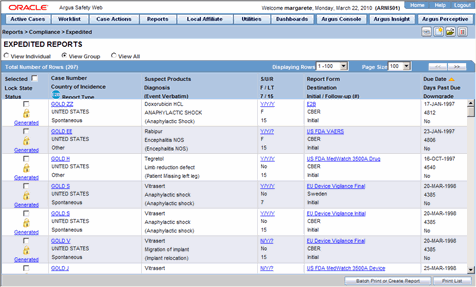
Reports Compliance And Aggregate Reports
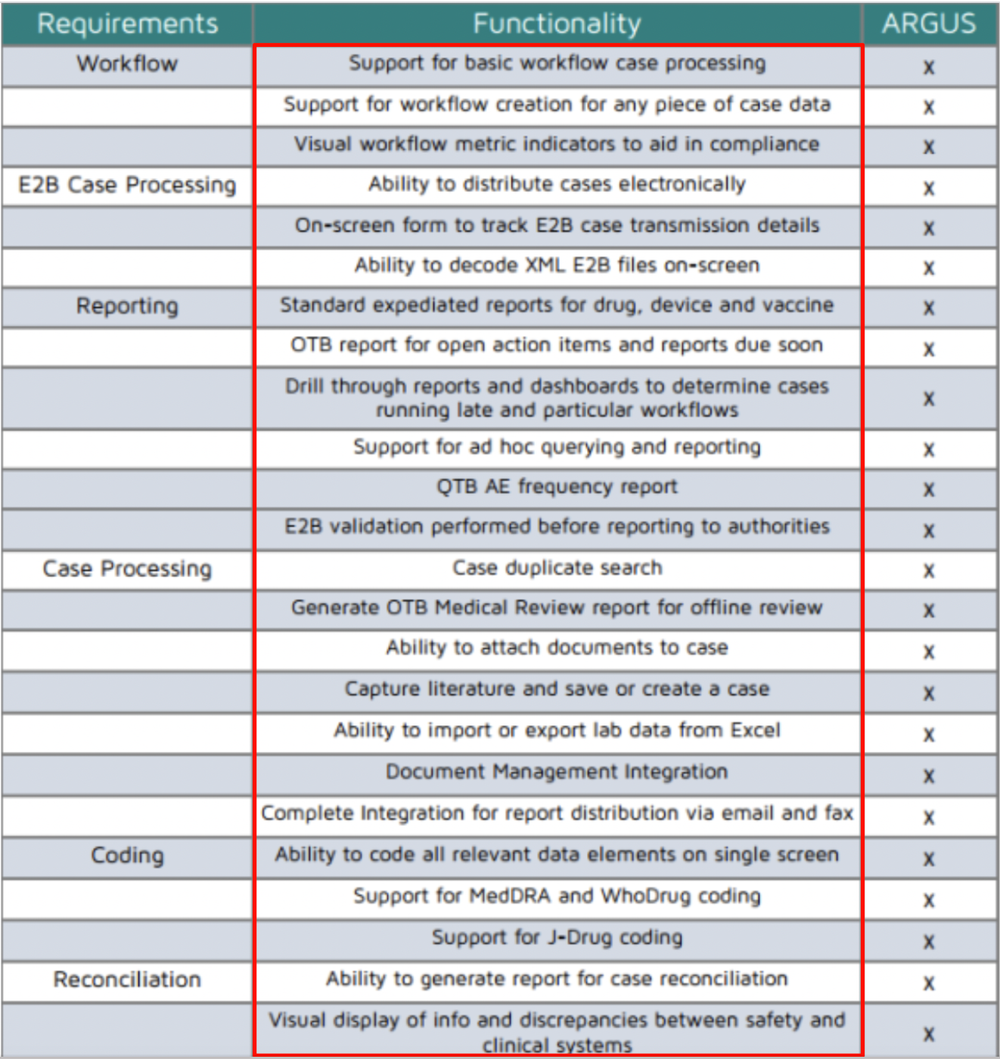
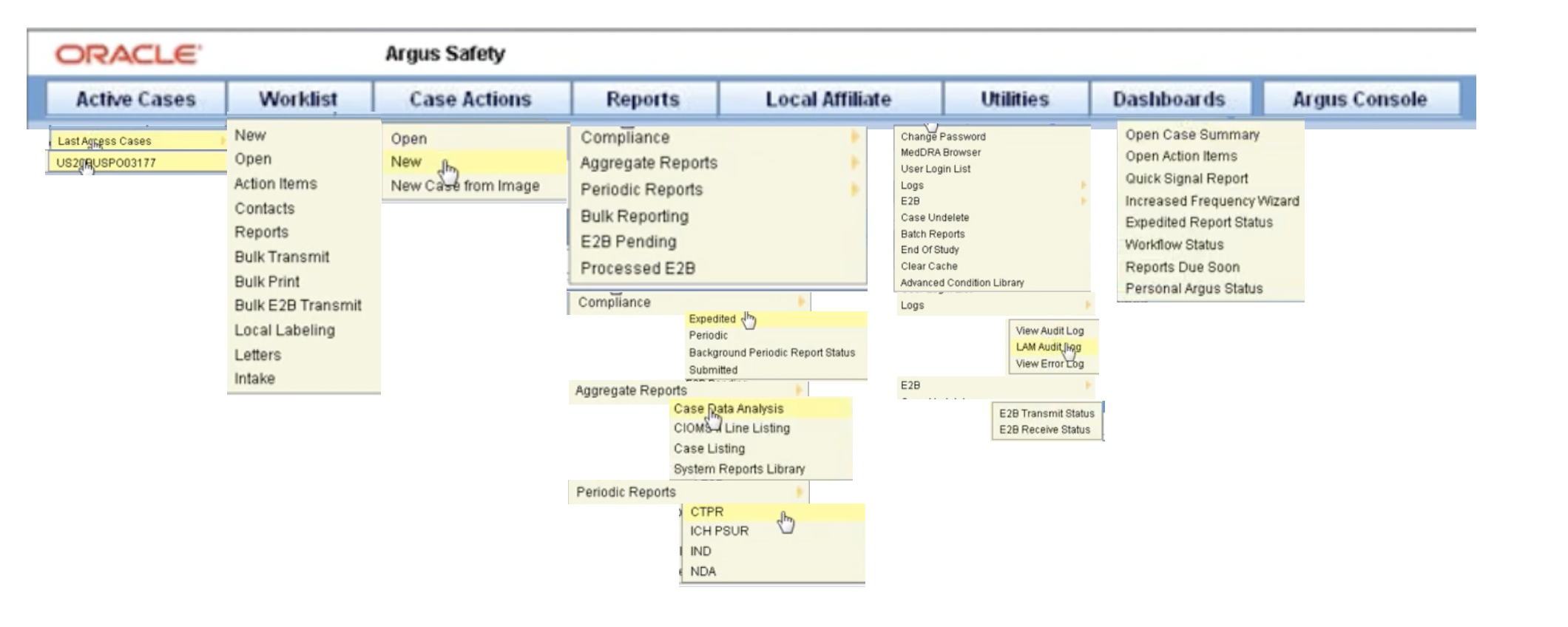
Posting Komentar untuk "Arisg Safety Database"