Arisg Safety Database Training
ARISg forms a core component of an integrated pharmacovigilance and risk management system enabling companies to monitor their products and identify safety risks proactively. A PPD safety physician then provided medical review of the reports.
Argus Software Training P Clinical Research Blog Certified Clinical Research Professionals Society Clinical Research Certification
Powerful extensible and user-friendly analytics and reports.
Arisg safety database training. It is the main repository for collected safety information for the companys products it facilitates the reporting of individual and aggregate safety data to authorities and third parties and provides a key source of information for ongoing detection of safety signals. Oversaw the hiring training and management of drug safety operations staff. Oracle Argus Safety Certification is a 6-part program that covers every tab and task needed to manage drug safety data when using the Oracle Argus Safety Training application.
A pharmacovigilance safety database is the central repository for individual case safety reports or ICSRs collected for a companys medicinal products from all sources globally. Pharmacovigilance work should be an exercise in accuracy timeliness and compliance and the software the safety database uses literally underpins all the other work staff perform. Have major involvement in the production of Aggregated Safety Reports.
Alternatively access to a safety database can be provided though a CRO or safety niche provider. The future of pharmacovigilance. ARISg facilitated quick and efficient generation of periodic reports within the system based on industry standard reporting requirements.
ARISg helps speed up the management of Adverse Drug reactions with the use of its configurable workflow and advanced automation features. PPD was able to assign a lead safety specialist for each report who compiled the data and wrote all sections of the report. Provide QC of PV Reports to ensure correct and accurate input of source data onto the global safety database ARISg.
Find Top Paid Free online arisg safety database training courses certifications trainings programs specialization at Naukri Learning. We have the experience of using the pre-validated Aris Global safety database ARISg a world leading Pharmacovigilance and clinical safety system. Arisg is used by most of the companies and argus is less when compared to it.
Learning Management System LMS demo recording of Argus Safety 60 training with content developed with Oracle User Productivity Kit UPKExample of functi. To obtain a Drug Safety Associate position that will promote growth stability and opportunity for advancement and has experience in responsible for overseeing specific aspects of adverse event processing. LifeSphere Safety is an end-to-end drug safety platform that helps hundreds of pharmacovigilance teams around the world save time and effort ensure future-proof compliance and unlock deep insights from safety.
Efficient case processing via automations and ease of use. Reduce Pharmacovigilance Safety Database Costs with our integrated Solution. The database owner may maintain the system on their servers or install the full system on a company server and allow access through licenses.
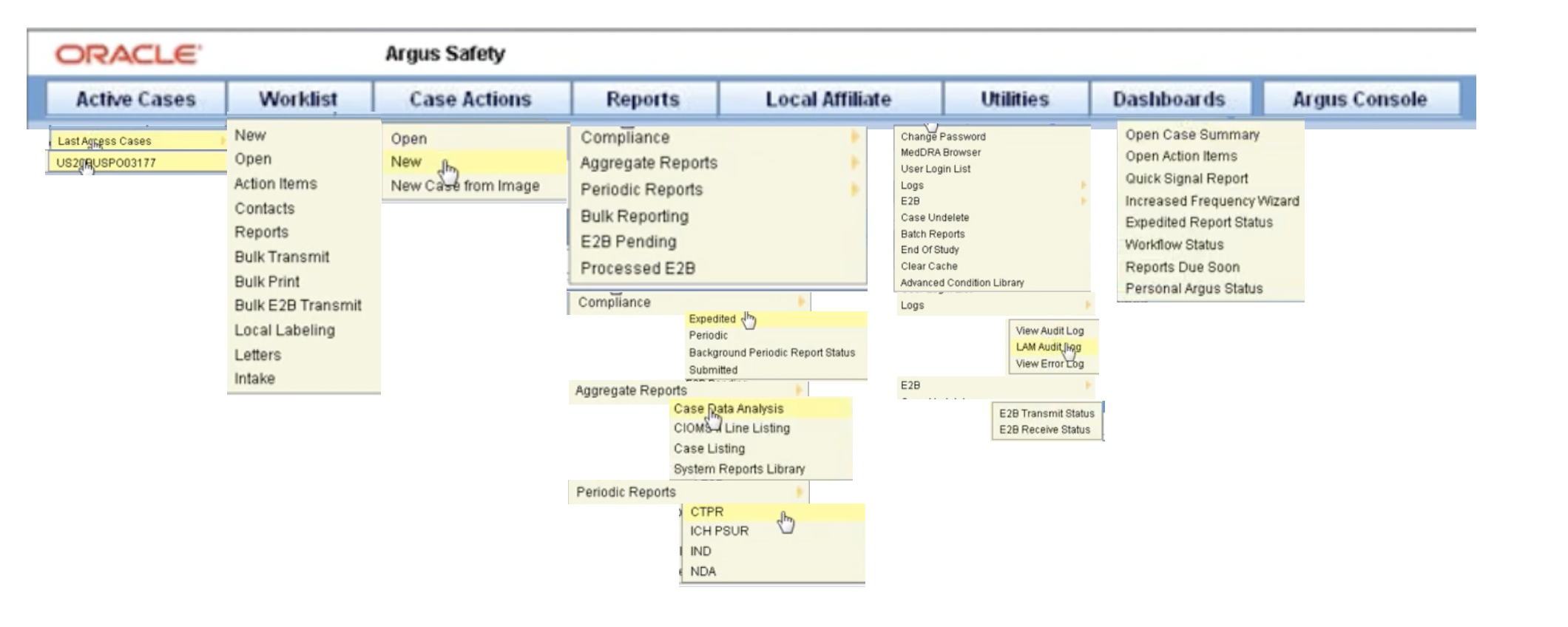
See a skim-through of our Argus Safety Database training below. So you may go for argus. Oracle Argus Safety Certification is a 6-part program that covers every tab and task needed to manage drug safety data when using the Oracle Argus Safety Training application.
See a skim-through of our Argus Safety Database training below. Most of the companies are upgrading from arisg to argus due to its simple and user friendly for expediting the reports in timelines. This guide to some key features of the ARISgTM safety database can help you understand what.
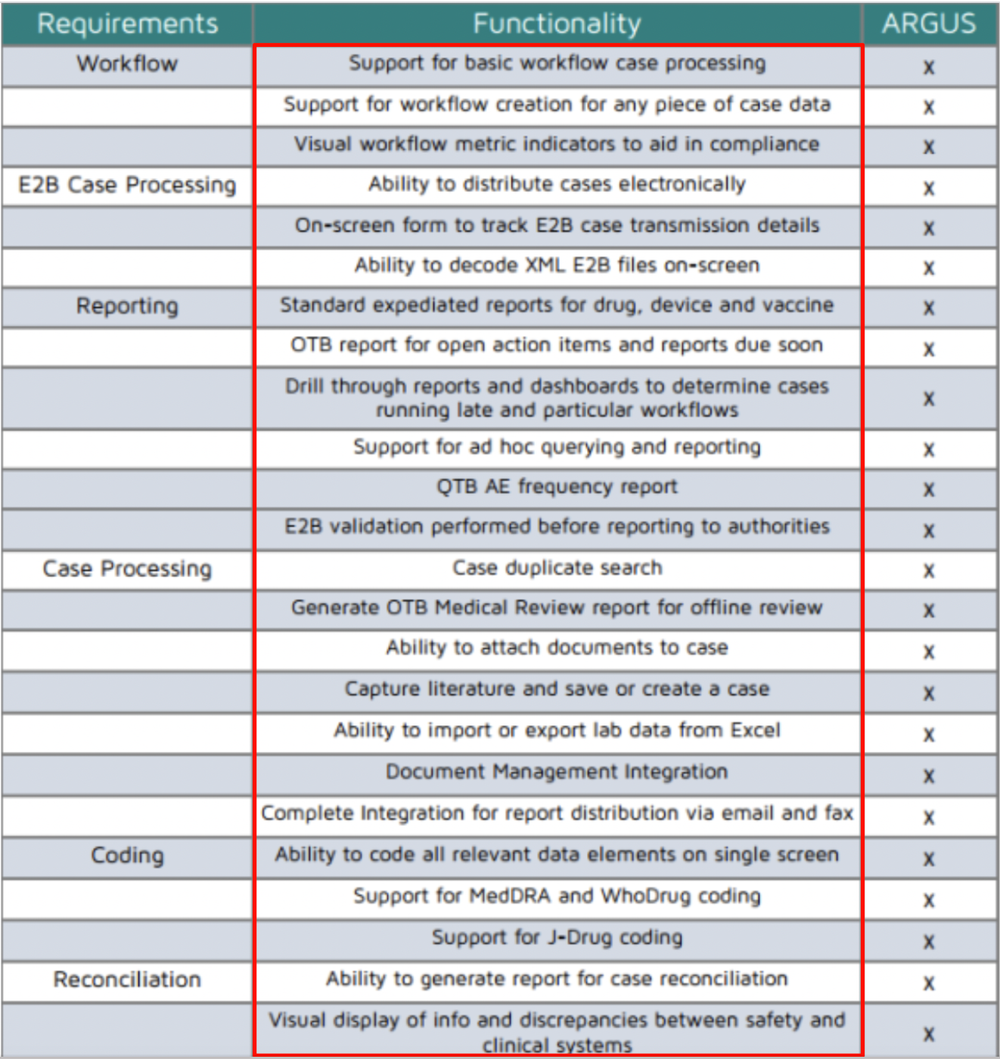
Any pharmacovigilance safety database must be deposited up to date with the most advanced regulatory requirements and certified to meet international standards and business requirements. It is vital that any pharmacovigilance safety database is kept up to date with the latest regulatory requirements and validated to meet both international standards and business requirements. Compare best arisg safety database training courses online from top Platforms Universities.
Compliance with global regulations and standards. Drug Safety Associate Company Name City State. End to end pharmacovigilance services including receipt processing and scientific analysis submission and distribution of Individual Case Safety Reports ICSRs Global and local literature management.
Argus is user-friendly and where as arisg is little complicated and tideous tool to complete the work. ISafety Multi-Tenant ARGUS SAFETY DATABASE Architecture. She is well-versed in MedDRA and WHO-Drug coding and knowledgeable in various safety databases ARISg Argus Trackwise with key leadership role in training and presenting drug and device safety.
For post-marketing drug safety surveillance Bioclinica provides. Without a reliable complaint and practical safety data base pharmacovigilance services simply wouldnt work in real life. Compare best arisg safety database training courses online from top Platforms Universities.
A Global Pharmacovigilance Safety Database sits at the heart of the vigilance system for medicinal products and devices. This may be a more attractive solution financially for limited case volumes. Lower cost and complexity through SaaS deployment.
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators. Global Safety Database Solution. This situation in Safety platforms space was not achieved overnight but rather over 5 7 years through market consolidation.
Responsible for day-to-day drug safety operations including the management of in-house Serious Adverse Event SAE case processing and external pharmacovigilance vendors. Prepare Business Objects searches listings for aggregated Safety Reports and ad hoc safety requests. Acted as the safety database ARISg.
More than Clinical I would say Safety is probably a better in example to highlight this situation where youd most likely find 2 products ruling the roost in Oracle Argus and Aris Globals ARISg LifeSphere Safety. ARGUS XPDite is an innovative rapid deployment system to operationalize Oracle Argus Safety platform within few weeks instead of months. Well help you stay ahead.
Our certification provides argus safety database training online which is internationally accepted for. Page 18 - Find Top Paid Free online arisg safety database training courses certifications trainings programs specialization at Naukri Learning. Aggregate report generation submission and distribution.
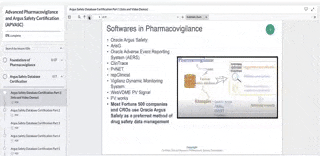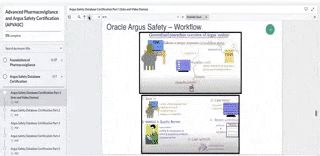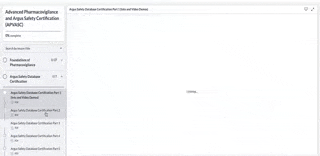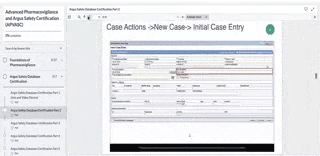
Single global database that includes Japan cases and. Horizon 3 Drug Safety Associate I Resume Objective. This offers the functionality to report and manage adverse events for drugs vaccines biologicals devices and combination products from case entry medical review coding via the integrated MedDRA browser to automatic generation of.
Corporate Training Program.

Oracle Aers Vs Argus Safety Youtube
Argus Software Training P Clinical Research Blog Certified Clinical Research Professionals Society Clinical Research Certification
Argus Software Training P Clinical Research Blog Certified Clinical Research Professionals Society Clinical Research Certification
Posting Komentar untuk "Arisg Safety Database Training"