What Does Database Lock Mean In Clinical Trials
Statistical checks for database acceptance before database lock of a clinical trial are not widely used. A database is locked after review query resolution and determination that it.
What is database lock in clinical trial.
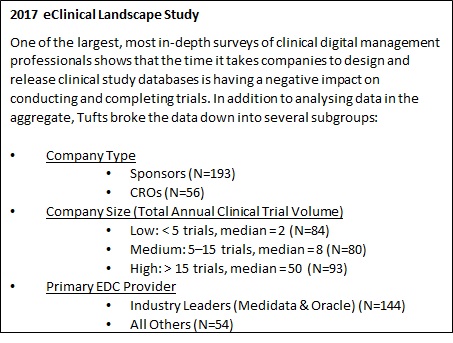
What does database lock mean in clinical trials. Additionally today interim analyses or analyses for safety review are more and more common and hence the teams need to work on unclean or semi-clean data. For companies that deliver the database after FPFV it takes roughly 75 longer to lock the study. What is a database lock.
It is done after a final quality check and data validation but before the data is extracted and formatted for submission. 4 Prevents unauthorized unintentional changes. At the completion of the clinical trial the clinical data manager ensures that all data expected to be captured has been accounted for and that all data management activities are complete.
Reconciling the databases to be sure that the clinical trial AEs are the same in the two databases is usually done periodically during the clinical trial rather than waiting till the end of the trial. Certainly you could have a 100 SDV in a NIS and perform the data cleaning for your interim analysis as you would for a clinical trial ie. Database lock is done once all data entered has been cleaned with no outstanding queries or discrepancies.
Clinical Data Collection Cleaning and Verification in Anticipation of Database Lock. Define Top Line Data. Database soft lock also known as database freeze is a milestone defined in the Statistical Analysis Report and is most commonly defined as the point where all case report form data has been input into the database and all known queries are resolved.
That is they have been recorded checked for accuracy etc. The quality of data generated plays. Database lockunlock - This is a controlled procedure to freeze data to prevent writeedit access to users of the system.
Closing or locking a study database is fundamental to preventing inadvertent or unauthorized changes once the final analysis and reporting of the data have begun. Database Lock means with respect to any Clinical Trial the date on which the databases containing the applicable clinical trial data is determined to be complete and locked in order to permit the analysis of the primary and secondary endpoints of such Clinical Trial. The impact of database-build delays is even greater by the time companies get to database lock.
This paper exploresthe implications of working with this dataand it presents tools and processes that can reduce their impact. Computer analysis of clinical trial data is certainly easier but no data collection method should begin to determine what information is not of interest. Within the conduct of a clinical trial there are many programming tasks that must be completed before data have been finalized and databases are locked.
Following the resolution of all queries the database can be finally locked and archived according to the procedures in the SOPsData handling protocols. At many instances after database lock an error or inconsistency is observed by either the medical writer or anyone from the clinical trial team involved in CSR writing. There are a number of instances when this is necessary.
A trial data manager plays a key role in achieving successful database lock by acting as liaison between statisticians clinical and technical personnel and ensuring that every step related to. Prepare the study database to be locked in anticipation of performing statistical analyses. However this is an unrealistic scenario in the vast majority of NIS.
Database lock A clinical trial term of art for an action taken to prevent further changes to the trial database. Visit Global Health Trials extensive Templates Library for relevant resources guidelines SOPs and checklists that. All data cleaning reconciliation and other data validation reports are performed on an ongoing basis until the end of study and final database lock.
Due to the fact that information can be studied at time of trial or later by other researchers all information gleaned from a trial should be kept intact. All such data will be collected in a database validated in accordance with 21 CFR Part 11 and with full audit trail. At this stage the data is declared final terminology varies but common descriptions are Database Lock and Database Freeze and the clinical data manager transfers data for statistical analysis.
Although important in open-label studies database closure is even more critical to preserve the integrity randomized trials after the blind has been broken. A synonym is data freeze. Data lock typically means that the data themselves in some sort of database are no longer being edited and are ready to be analyzed.
The database lock is one of the final steps taken during a clinical trial before submitting the information to the FDA. To create a final version. This corrects errors in near real-time and avoids late expedited reports to health authorities.
Lock the data and unlock upon request only. 3 2 1 PI signs all data from the beginning until the end which is then entered in eCRFs. So what does this mean.
Means with respect to a clinical study a summary of demographic data the data for the primary endpoint and a summary of safety data which are based upon an unblinded locked database. Clinical trial is intended to find answers to the research question by means of generating data for proving or disproving a hypothesis. Also all external data such as the safety lab database or other vendor databases should have been cleaned and.
Orchestrated Clinical Trials Iqvia

Data Management George Clinical

Impact Of Data Management On Clinical Trials New Study

Posting Komentar untuk "What Does Database Lock Mean In Clinical Trials"